1. Switchable-Polarity Solvents (SPS)
These have low polarity until they are exposed to an atmosphere of CO2, at which point they change into high-polarity ionic liquids. The polarity difference is large enough that many solutes are soluble in only one form of the solvent. The process is reversed by removal of the CO2 from solution. These liquids are also extremely efficient greenhouse gas-capture agents.
Key references are listed below.
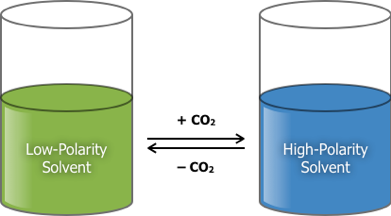
2. Switchable-Hydrophilicity Solvents (SHS)
These are liquid solvents that are normally so hydrophobic that they have very little miscibility with water and form a biphasic mixture when mixed with water. However, when exposed to CO2, these solvents become very hydrophilic and completely miscible with water. Therefore these solvents can behave like hexane but be easily removed, by extraction with carbonated water, without distillation.
Key references are listed below.
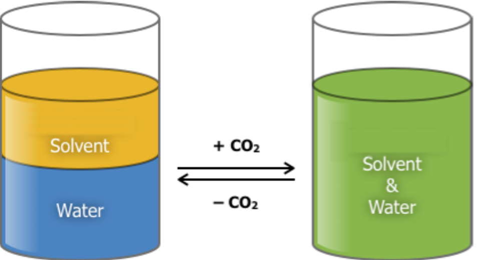
3. Switchable Water (SW)
Switchable water is an aqueous solution containing a water-soluble amine or polyamine. The solution has a very low ionic strength under air but when CO2 is introduced the ionic strength jumps greatly due to the formation of bicarbonate salts. These solutions can be used for many applications including:
a) Extractions: Organic compounds can be dissolved in the CO2-free version and then expelled by the addition of CO2.
b) Water purification: The CO2-free version has a lower osmotic pressure than the version with CO2, making it a useful draw solution for forward osmosis recovery of water from wastewater and seawater. (See research page about Water).
c) Breaking emulsions and suspensions: Non-switchable surfactants, such as those traditionally used in industry, will stabilize emulsions and suspensions in the CO2-free version but will fail once CO2 is added.
Switchable Water is is being commercialized by Forward Water Technologies. http://www.forwardwater.com
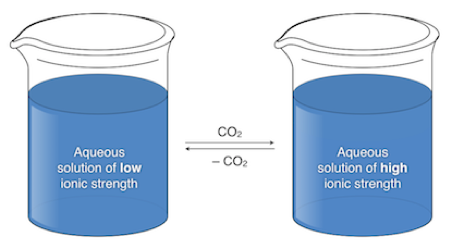
References
About Switchable-Polarity Solvents (SPS):
- A. R. Boyd, P. G. Jessop, J. M. Dust, E. Buncel, “Switchable polarity solvent (SPS) systems: probing solvatoswitching with a spiropyran (SP) – merocyanine (MC) photoswitch”, Organic & Biomolecular Chem. 2013, 11, 6047-6055.
- D. J. Heldebrant, P. K. Koech, T. Ang, C. Liang, J. E. Rainbolt, C. R. Yonker, and P. G. Jessop, “Reversible Zwitterionic Liquids, The Reaction of Alkanol Guanidines, Alkanol Amidines, and Diamines With CO2“, Green Chemistry, 2010, 12, 713-721.
- P. G. Jessop, D. J. Heldebrant, L. Xiaowang, C. A. Eckert, C. L. Liotta, Nature, 2005, 436, 1102.
- L. Phan, J. R. Andreatta, L. K. Horvey, C. F. Edie, A.-L. Luco, A. Mirchandi, D. J. Darensbourg, P. G. Jessop, J. Org. Chem., 2008, 73, 127.
- L. Phan, X. Li, D. J. Heldebrant, R. Wang, D. Chiu, E. John, H. Huttenhower, P. Pollet, C. A. Eckert, C. L. Liotta, P. G. Jessop, Ind. Eng. Chem. Res., 2008, 47, 539.
- P. M. Mathias, K. Afshar, F. Zheng, M. D. Bearden, C. J. Freeman, T. Andrea, P. K. Koech, I. Kutnyakov, A. Zwoster, A. R. Smith, P. G. Jessop, O. G. Nik, and D. J. Heldebrant, “Improving the Regeneration of CO2-Binding Organic Liquids with a Polarity Change”, Energy Env. Sci., 2013, 6, 2233-2242.
- D. J. Heldebrant, P. K. Koech, T. Ang, C. Liang, J. E. Rainbolt, C. R. Yonker, and P. G. Jessop, “Reversible Zwitterionic Liquids, The Reaction of Alkanol Guanidines, Alkanol Amidines, and Diamines With CO2”, Green Chemistry, 2010, 12, 713-721.
- D. J. Heldebrant, C. R. Yonker, P. G. Jessop, L. Phan, “Reversible Uptake of COS, CS2 and SO2: Ionic liquids with O-alkylxanthate, O-alkylthiocarbonyl and O-alkylsulfite Anions”, Chem. Eur. J., 2009, 15, 7619-7627.
- D. J. Heldebrant, C. R. Yonker, P. G. Jessop, L. Phan, “Organic liquid CO2 Capture Agents with High Gravimetric CO2 Capacity,” Energy & Environ. Science, 2008, 1, 487-493.
- P. G. Jessop, D. J. Heldebrant, X. Li, C. A. Eckert and C. L. Liotta, “Reversible Nonpolar-to-Polar Solvent” Nature, 2005, 436, 1102.
About Switchable-Hydrophilicity Solvents (SHS):
- H. Poole, J. Gauthier, J. Vanderveen, P. G. Jessop, R. Lee, “Use of a switchable-hydrophilicity solvent as both solvent and catalyst in aldol condensation”, Green Chem., 2019, 21, 6263-6267
- K. Viner, H. M. Roy, R. Lee, O. He, P. Champagne, P. G. Jessop, “Transesterification of soybean oil using a switchable-hydrophilicity solvent, 2-(dibutylamino)ethanol”, Green Chem., 2019, 21, 4786-4791.
- A. Cicci, G. Sed, P. G. Jessop, M. Bravi, “A novel switchable-hydrophilicity, natural deep eutectic solvent (NaDES)-based system for bio-safe biorefinery”, RSC Adv., 2018, 8, 37092-37097.
- J. R. Vanderveen, J. Geng, S. Zhang, P. G. Jessop, “Diamines as switchable-hydrophilicity solvents with improved phase behaviour”, RSC Adv., 2018, 8, 27318-27325.
- A. Cicci, G. Sed, P. G. Jessop, M. Bravi, “Circular Extraction: An Innovative Use of Switchable Solvents for the Biomass Biorefinery”, Green Chem., 2018, 20, 3908-3911.
- X. Su, P. G. Jessop, M. F. Cunningham, “Preparing Artificial Latexes Using a Switchable Hydrophilicity Solvent”, Green Chem., 2017, 19, 1889-1894.
- J. Großeheilmann, J. R. Vanderveen, P. G. Jessop and U. Kragl, “Switchable-Hydrophilicity Solvents for Product Isolation and Catalyst Recycling in Organocatalysis”, ChemSusChem, 2016, 9, 696-702.
- J. R. Vanderveen, R. I. Canales, Y. Quan, C. Chalifoux, M. A. Stadtherr, J. F. Brennecke and P. G. Jessop, “Non-random two-liquid modelling of switchable-hydrophilicity solvent systems: N,N-dimethylcyclohexanamine, water, and toluene”, Fluid Phase Equilibria, 2016, 409, 150-156.
- J. Vanderveen, L. Patiny, C. Chalifoux, M. J. Jessop, P. G. Jessop, “A Virtual Screening Approach to Identifying the Greenest Compound for a Task: Application to Switchable-Hydrophilicity Solvents”, Green Chem., 2015, 17, 5182-5188.
- J. Durelle, J. R. Vanderveen, Y. Quan, C. Chalifoux, J. E. Kostin, P. G. Jessop, “Extending the Range of Switchable-Hydrophilicity Solvents”, PhysChemChemPhys, 2015,17, 5308-5313.
- J. Durelle, J. R. Vanderveen, P. G. Jessop, “Modelling the Behaviour of Switchable-Hydrophilicity Solvents”, PhysChemChemPhys, 2014, 16, 5270-5275.
- J. R. Vanderveen, J. Durelle, P. G. Jessop, “Design and Evaluation of Switchable-Hydrophilicity Solvents”, Green Chem., 2014, 16, 1187-1197.
- D. Fu, S. Farag, J. Chaouki, P. G. Jessop, “Extraction of phenols from lignin microwave-pyrolysis oil using a switchable hydrophilicity solvent”, Bioresource Tech., 2014, 154, 101-108.
- A. Holland, D. Wechsler, A. Patel, B. M. Molloy, A. R. Boyd and P. G. Jessop, “Separation of Bitumen from Oil Sands using a Switchable Hydrophilicity Solvent”, Can. J. Chem., 2012, 90, 805-810.
- A. R. Boyd, P. Champagne, P. J. McGinn, K. M. MacDougall, J. E. Melanson, and P. G. Jessop, “Switchable Hydrophilicity Solvents for Lipid Extraction from Microalgae for Biofuel Production”, Bioresource Technology, 2012, 118, 628-632.
- P. G. Jessop, L. Kozycz, Z. Ghoshouni Rahami, D. Schoenmakers, A. R. Boyd, D. Wechsler, and A. M. Holland, “Tertiary Amine Solvents having Switchable Hydrophilicity”, Green Chem., 2011, 13, 619-623.
- P. G. Jessop, L. Phan, A. Carrier, S. Robinson, C. J. Dürr and J. R. Harjani, “A Solvent having Switchable Hydrophilicity”, Green Chemistry, 2010, 12, 809-814.
About Switchable Water (SW):
- J. R. Vanderveen, S. Burra, J. Geng, A. Goyon, A. Jardine, H. E. Shin, Tamer Andrea, • P. J. Dyson, and P. G. Jessop, “Characterizing the Effects of a Switchable Water Additive on the Aqueous Solubility of Small Molecules”, ChemPhysChem, 2018, 19, 2093-2100.
- X. Yuan, B. Richter, K. Jiang, K. Boniface, A. Cormier, C. Sanders, C. Palmer, P. G. Jessop, M. Cunningham, R. Oleschuk, “Carbonated water for the separation of carboxylic compounds: a chromatography approach”, Green Chem., 2018, 20, 440-448.
- X. Yuan, E. G. Kim, C. A. Sanders, B. E. Richter, M. F. Cunningham, P. G. Jessop, and R. D. Oleschuk, “CO2 Modified Solvents for Chromatographic Separation”, Green Chem., 2017, 19, 1757-1765.
- A. K. Alshamrani, J. Vanderveen, P. G. Jessop, “A Guide to the Selection of Switchable Functional Groups for CO2-Switchable Compounds”, PhysChemChemPhys, 2016, 16, 19276-19288.
- S. Jahangiri, S. Mercer, P. Jessop, G. Peslherbe, “Computational Investigation of the Hydration of Alkyl Diammonium Chlorides and their Effect on THF/Water Phase Separation”, J. Phys. Chem. B, 2013, 117, 8010-8017.
- X. Su, M. Cunningham, P. G. Jessop, “Use of a Switchable Hydrophobic Associative Polymer to Create an Aqueous Solution of CO2-Switchable Viscosity”, Polymer Chem. 2014, 5, 940-944.
- X. Su, M. F. Cunningham, and P. G. Jessop, “Switchable Viscosity Triggered by CO2 using Smart Worm-like Micelles in Water”, Chem. Commun., 2013, 49, 2655-2657.
- X. Su, T. Robert, S. M. Mercer, C. Humphries, M. F. Cunningham and P. G. Jessop, “Normal Surfactants Can be Made Stimuli-Responsive with “Switchable Water”: Destabilization and Re-stabilization of Anionic Latexes and Emulsions Triggered by CO2”, Chem Eur. J., 2013, 19, 5595-5601.
- C.-S. Chen, Y. Y. Lau, S. M. Mercer, T. Robert, J. H. Horton and P. G. Jessop “The Effect of Switchable Water Additives on Clay Settling”, ChemSusChem, 2013, 6, 132-140.
- T. Robert, S. M. Mercer, T. J. Clark, B. E. Mariampillai, P. Champagne, M. F. Cunningham, and P. G. Jessop “Nitrogen-Containing Polymers as Potent Ionogens for Aqueous Solutions of Switchable Ionic Strength: Application to Separation of Organic Liquids and Clay Particles from Water”, Green Chem., 2012, 14, 3053-3062.
- S. M. Mercer, T. Robert, D.V. Dixon, and P. G. Jessop “Recycling of a Homogeneous Hydroformylation Catalyst using Switchable Water”, Catal. Sci. Technol., 2012, 2, 1315-1318.
- S. M. Mercer, T. Robert, D. V. Dixon, C.-S. Chen, Z. Ghoshouni, J. R. Harjani, S. Jahangiri, G. H. Peslherbe and P. G. Jessop “Design, Synthesis, and Solution Behaviour of Small Polyamines as Switchable Water Additives”, Green Chem., 2012, 14, 832-839.
- Jessop, Mercer, Brown and Robert, “Water of Switchable Ionic Strength”, US Patent application US 13/578,290, filed 2011.
- S. M. Mercer, P G. Jessop, “Switchable Water: Aqueous Solutions of Switchable Ionic Strength”, ChemSusChem, 2010, 3, 467-470.
