Algae is a rapidly-growing biomass source of lipids and polysaccharides. The lipids can be converted into biodiesel but this is only practical if the entire process doesn’t take too much energy. The polysaccharides from algae and other biomass can be converted into platform chemicals and jet fuel. Lignin, on the other hand, is a great source of aromatic chemicals, but both algae and lignin have significant practical problems primarily associated with separations. Lignin is a nonrepeating highly crosslinked polymer containing many aromatic rings. Lignin can be pyrolyzed or depolymerized to give oils that contain valuable aromatic compounds that could potentially be used as platform chemicals.
1. Separation of lipids from algae
The problematic steps for algae are the three separation steps:
a) algae from water,
b) algae lipids from the algae cells, and
c) extraction solvent from the lipids.
Each of these steps can be very energetically costly if not designed well. We have found that the addition of compressed CO2 gas causes algae cells to coagulate and separate from the bulk water. Once algae has been dewatered, the extraction of the lipids from the algae can be achieved by almost any organic solvent, but the subsequent separation of the lipids from the organic solvent can be difficult and energy-intensive. We have found that switchable-hydrophilicity solvents can be used to solve this issue, without any need for distillation or high pressure. Also, liquid CO2 can be used and easily removed afterwards. Liquid CO2 doesn’t require the very high pressures of supercritical CO2, works at room temperature, and is very selective for neutral lipids. Even better, liquid CO2 works even if the algae has not been fully dried, which saves a lot of energy.
This research is being performed in collaboration with Prof. Champagne of Civil Engineering at Queen’s.
Key references are listed below.
2. Phenols from lignin pyrolysis oil
Pyrolysis breaks down lignin into an oil that contains many single-ring phenols in addition to heavier material. The single-ring phenols have the most potential value as feedstock or platform chemicals because few other abundant types of biomass feedstocks contain aromatic rings. However, the separation of the single-ring phenols from the heavier materials in the oil is difficult. We have found two methods of achieving this separation, a) switchable-hydrophilicity solvents, and b) supercritical fluid rectification. The latter process gives the cleanest separation. We can also convert the phenols into two classes of biomass-derived solvents: anisoles and methyl cyclohexyl ethers.
Much of this research has been performed in collaboration with Prof. J. Chaouki of École Polytechnique de Montréal.
Key references are listed below.
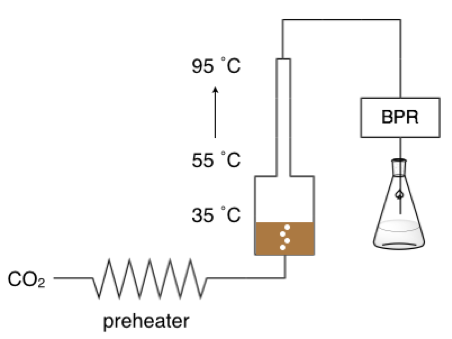
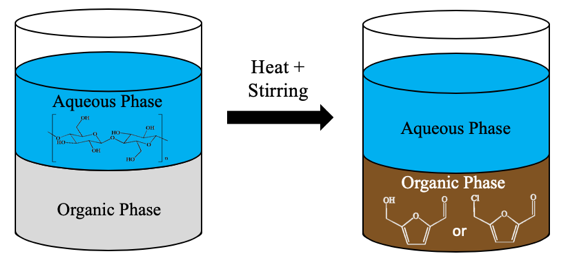
3. Conversion of Polysaccharides to Platform Chemicals
Polysaccharides and biomass sources, such as cellulose, wood species and macroalgae, can be converted into intermediate-like platform molecules. Platform molecules are versatile molecules of interest that can be further converted into a variety of useful products, such as polymers, fuels, specialty chemicals and pharmaceuticals. Our group primarily studies 5-hydroxymethylfurfural (HMF) and 5-chloromethylfurfural (CMF), which can each be produced through dehydration reactions (and a chlorination step in the case of CMF) of glucose, and therefore polysaccharides that contain glucose molecules. We have used of CO2 as an acid catalyst in the production of HMF to achieve improved HMF yields from carbohydrates while using CO2 instead of stronger acids. In the case of CMF, alternative methods and solvents for CMF synthesis have been used instead of the typical procedures that use large volumes of chlorinated solvents to achieve high CMF yields.
This research is being performed in collaboration with Prof. Champagne of Civil Engineering at Queen’s.
References
About Lipids from Algae
- K. Viner, P. Champagne, P. G. Jessop, “Comparison of cell disruption techniques prior to lipid extraction from Scenedesmus species slurries for biodiesel production using liquid CO2”, Green Chem. (2018) 20, 4330-4338.
- J. Harris, K. Viner, P. Champagne, P. G. Jessop, “Advances in microalgae lipid extraction for biofuel production: a review”, Biofuels, Bioproducts & Biorefining (2018) 12, 1118-1135.
- R. Lee, P. G. Jessop, P. Champagne, “CO2 pressure-induced coagulation of microalgae”, Philosophical Trans. Royal Society: A (2015), accepted.
- A. Paudel, M. J. Jessop, S. H. Stubbins, P. Champagne, and P. G. Jessop, “Extraction of Lipids from Microalgae using CO2-expanded Methanol and Liquid CO2”, Bioresource Technology (2015) 184, 286-290.
- A. R. Boyd, P. Champagne, P. J. McGinn, K. M. MacDougall, J. E. Melanson, and P. G. Jessop “Switchable Hydrophilicity Solvents for Lipid Extraction from Microalgae for Biofuel Production”, Bioresource Technology (2012) 118, 628-632.
- S. Ge, P. Champagne, H. Wang, P. G Jessop, and M. F. Cunningham, “Microalgae Recovery from Water for Biofuel Production under Environmentally Relevant Conditions Using CO2-Switchable Crystalline Nanocellulose”, Env. Sci. Technol. (2016) 50, 7896-7903.
- R. Lee, P. G. Jessop, P. Champagne, “CO2 pressure-induced coagulation of microalgae”, Philosophical Trans. Royal Society: A (2015), 373, 20150016; DOI: 10.1098/rsta.2015.0016.
- A. Paudel, M. J. Jessop, S. H. Stubbins, P. Champagne, and P. G. Jessop, “Extraction of Lipids from Microalgae using CO2-expanded Methanol and Liquid CO2”, Bioresource Technology (2015) 184, 286-290.
- B. P. Mudraboyina, D. Fu, and P. G. Jessop, “Supercritical Fluid Rectification of Lignin Microwave-Pyrolysis Oil”, Green Chem. (2015) 17, 169-172.
About Phenols from Lignin
- B. P. Mudraboyina, D. Fu, and P. G. Jessop, “Supercritical Fluid Rectification of Lignin Microwave-Pyrolysis Oil”, Green Chem. (2015) 17, 169-172.
- S. Farag, D. Fu, P. G. Jessop, J. Chaouki, “A Detailed Compositional Analysis and Structural Investigation of a Bio-oil from Microwave Pyrolysis of Kraft Lignin”, J. Anal. Appl. Pyrolysis, (2014) 109, 249-257.
- D. Fu, S. Farag, J. Chaouki, P. G. Jessop, “Extraction of phenols from lignin microwave-pyrolysis oil using a switchable hydrophilicity solvent”, Bioresource Tech., (2014) 154, 101-108.
- S. Farag, B. P. Mudraboyina, P. G. Jessop, and J. Chaouki, “Impact of the Heating Mechanism on the Yield and Composition of a Bio-oil from Pyrolysis of Kraft Lignin”, Biomass & Bioenergy (2016) 95, 344-353.
- B. P. Mudraboyina, S. Farag, A. Banerjee, P. G. Jessop and J. Chaouki, “Supercritical Fluid Rectification of Lignin Pyrolysis Oil Methyl Ether (LOME) and Its Use as a Bio-derived Aprotic Solvent”, Green Chem. (2016) 18, 2089-2094.
- S. Farag, D. Fu, P. G. Jessop, J. Chaouki, “A Detailed Compositional Analysis and Structural Investigation of a Bio-oil from Microwave Pyrolysis of Kraft Lignin”, J. Anal. Appl. Pyrolysis, (2014) 109, 249-257.
- D. Fu, S. Farag, J. Chaouki, P. G. Jessop, “Extraction of phenols from lignin microwave-pyrolysis oil using a switchable hydrophilicity solvent”, Bioresource Tech., (2014) 154, 101-108.
About Conversion of Polysaccharides
- R. Lee, J. Harris, P. Champagne, P. G. Jessop, “CO2-catalysed conversion of carbohydrates to 5-hydroxymethyl furfural”, Green Chem. (2016) 18, 6305-6310.
- R. Lee, J. R. Vanderveen, P. Champagne, P. G. Jessop, “CO2-catalysed aldol condensation of 5-hydroxymethylfurfural and acetone to a jet fuel precursor”, Green Chem. (2016) 18, 5118-5121.
- S. Siankevich, Z. Fei, R. Scopelliti, P. Jessop, J. Zhang, N. Yan, and P. J. Dyson, “Direct Conversion of Mono- and Polysaccharides into 5-Hydroxymethylfurfural Using Ionic-Liquid Mixtures”, ChemSusChem (2016) 9, 2089-2096.
